According to the research data of the International diabetes Federation (IDF), the number of global adult diabetes patients will be about 537 million by 2021, and it is estimated that this number will increase by about 46% to 783 million by 2045! Obviously, diabetes has become one of the main threats to human health.
For patients with diabetes, "sugar control" is an unavoidable routine. However, many sugar enthusiasts know that this path of controlling sugar is not easy. In traditional medical methods, blood glucose monitoring and corresponding treatment are carried out in professional medical institutions, which undoubtedly consumes a lot of costs and time for patients, and is also one of the important reasons why "sugar control" is difficult to sustain and the effect is unsatisfactory in reality.
Technological Development of Blood Glucometer
However, with the increasing awareness of people's health and the development of modern technology, this situation is changing. More and more medical devices are gradually abandoning their professional status and transforming into consumer grade products, making them accessible to ordinary people in their daily lives. A blood glucose meter is one of them.
The emergence of household blood glucose monitors allows ordinary people to independently monitor their blood glucose levels anytime and anywhere, and to monitor their health status in real-time. Especially with the rise of the Internet of Things, blood glucose meters have become increasingly intelligent. They can transmit blood glucose monitoring data to mobile apps and the cloud through wireless communication, connect to smart medical systems, and provide personalized treatment recommendations based on patient data.
It is worth noting that in recent years, the evolution of blood glucose meters has shown a new trend, namely the emergence of continuous blood glucose monitoring (CGM) and the rise of the market.
The traditional finger blood testing (BGM) method collects blood by puncturing fingers or other body parts to complete blood glucose testing. Although this method is "classic", it also has many drawbacks, such as: the invasive blood collection process is more painful, and improper operation may lead to wound infection; The operation is relatively cumbersome, and there are many tools and materials required for blood glucose testing (blood collection pens, test strips, testing instruments, etc.); The monitoring frequency is limited, making it impossible to monitor continuous changes in blood sugar, and there may be blind spots in monitoring... In order to make up for the shortcomings of the BGM method mentioned above, people have developed CGM technology.
As the name suggests, "Continuous Blood Glucose Monitoring (CGM)" is the ability to continuously collect blood glucose data, monitor blood glucose fluctuations throughout the time period, and ensure that "sugar control" does not leave blind spots. Compared to BGM, this is clearly a significant advantage. The principle of CGM is not complicated. It is a technology that indirectly measures blood sugar by recording the electrical signals generated by glucose oxidation reaction in the interstitial fluid through microelectrodes implanted in subcutaneous tissue. With only a one-time "minimally invasive" installation of sensor probe patches, it can work continuously for a long time, reducing patient pain and making use more convenient.
It is precisely because of these characteristics that, CGM is highly favored by the market. According to data, from 2015 to 2020, the global CGM market size rapidly increased from $1.7 billion to $5.7 billion, with an annual compound growth rate of up to 28.2%. Moreover, in many markets (such as China), The penetration rate of CGM blood glucose meters is not high, and the future market prospects are worth looking forward to.

Figure 1: Continuous blood glucose monitoring (CGM) is on the rise
-
It can be seen that in the long fight against diabetes, technological progress is giving us more and more initiative; In another sense, this will also accelerate the iteration of blood glucose meter products, making them a star product in the field of intelligent medical devices. According to research data from QYResearch, the global market for self monitoring and continuous blood glucose monitoring is expected to reach $27.3 billion by 2029, with a compound annual growth rate of 10.2% in the coming years.
Crossing the technical threshold of blood glucose metersFor players in the medical electronics market, the market cake of blood glucose meters is indeed tempting, but there are still several hurdles that cannot be avoided during research and development if you want to eat it.
Firstly, due to the fact that blood glucose meters belong to medical devices, there are special requirements for the accuracy, precision, reliability, and safety of product measurements, which require compliance with specific industry standards. This is undoubtedly a threshold.
Secondly, as a high-frequency medical device, the portability and wearability of blood glucose meters are the trend, and miniaturization design is a significant challenge for any electronic system.
At the same time, portability favors battery power supply, which also places higher demands on the low power consumption characteristics of blood glucose meters.
Furthermore, the consumerism of medical devices will certainly expand the market cake, but ordinary consumers are also more sensitive to costs, so they must also do a good job in cost optimization during product development.
Finally, as the level of intelligence increases, the functions of blood glucose meters will also become more diverse - for example, wireless connectivity has become a standard feature in mainstream products - and the system will become more complex, requiring developers to face greater workload.
It is not difficult to see from the above factors that developing a commercial blood glucose meter product is not an easy task. To successfully overcome these technological barriers, a key point is to carefully control the upstream of product research and development, the selection and application of components, and select highly reliable materials to build the technical foundation of the product.
Of course, there is a huge business opportunity in the blood glucose meter market, and component manufacturers have also recognized it. Therefore, they have been following the development of technology and the market, making meticulous product layouts to better meet the needs of developers.
In this field, Murata Manufacturing has a very comprehensive technical preparation, relying on a rich product portfolio to create a comprehensive solution around blood glucose meters (including CGM products).Provide supporting electronic components (Provide supporting electronic components (Murata MLCC (GRM), Murata SMT inductor LGQ, LQP)
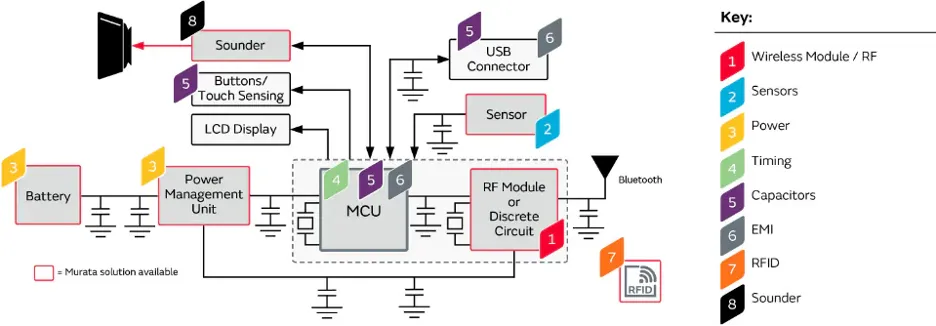
Figure 2: Murata blood glucose meter solution
In this article, we will focus on recommending Murata's three products for blood glucose meters. As you can see, these products can well meet the design requirements of blood glucose meters, making it easy for developers to cope with challenges.
Bluetooth low-power module: creating smart connections
With the improvement of the intelligence level of blood glucose meters, Bluetooth low-power (BLE) wireless connection has become the standard configuration of products, which can connect blood glucose meters with mobile phones, tablets PDA and other intelligent terminals are connected together to transmit the patient's blood glucose data, or to complete the debugging, configuration, and OTA functions of the blood glucose meter.
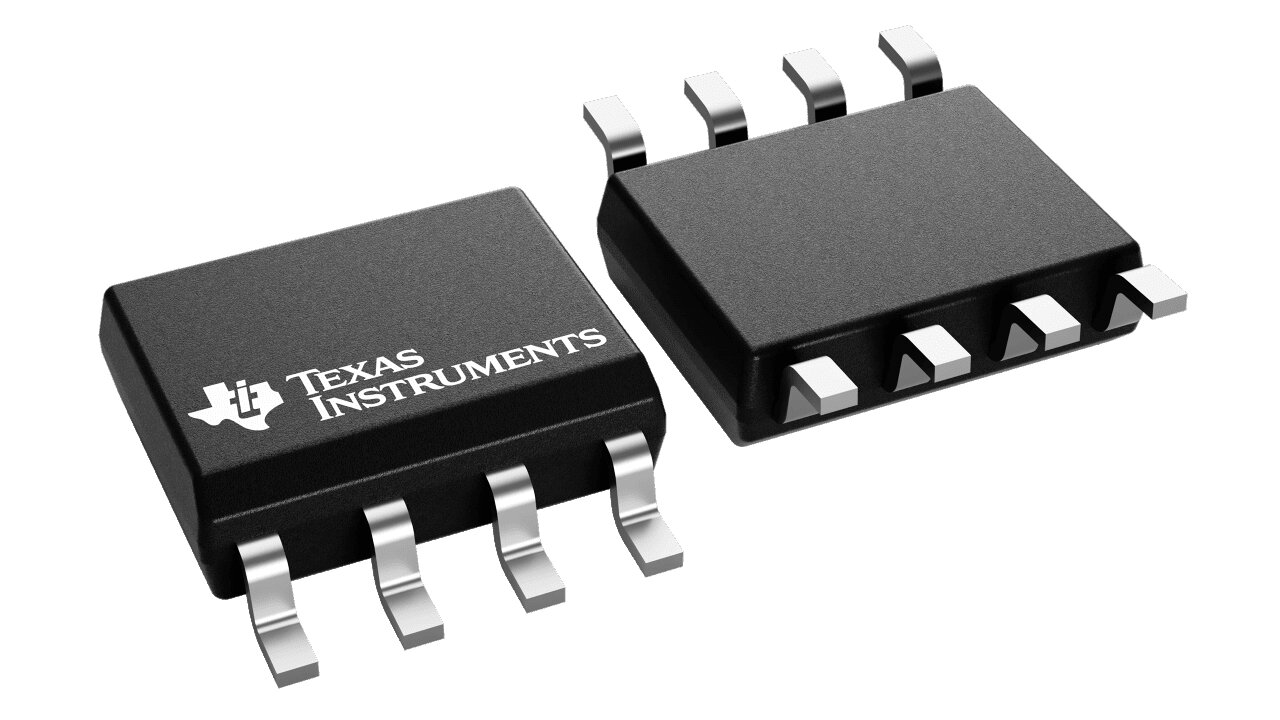
Based on the design requirements of the blood glucose meter mentioned earlier, it is not difficult to see that small size, low power consumption, and high-quality connection are the core requirements for the BLE module in blood glucose meter products. Murata's MBN52832 Bluetooth low-power 5.0 module is carefully crafted for this type of ultra-low power data communication application.

Figure 3: MBN52832 BLE 5.0 Module
The MBN52832 BLE module integrates Nordic's BLE SoC, RF front-end, and crystal into an ultra small form factor, which can well meet the design requirements for miniaturization of blood glucose meters.
The core of the module is Nordic's nRF52832 wireless SoC, which complies with the BLE 5.0 specification and can provide higher throughput, larger broadcast capacity, and improved Channel Coexistence Algorithm (SCA), providing advanced wireless connection characteristics. This wireless SoC is equipped with an Arm Cortex-M4 core, which integrates 64KB RAM, 512KB flash memory, and rich interfaces. It is suitable for various IoT applications and can support the expansion of blood glucose meter functions.
This module provides two Bluetooth antenna configuration options: using onboard PCB antennas or connecting external antennas through pin pads, which brings greater flexibility to the design. The MBN52832 BLE module has also obtained SIG certification, as well as FCC Regulatory certifications such as IC and ETSI help reduce the workload of system designers and shorten product launch time, making them highly suitable for small battery powered medical and health applications such as blood glucose meters.
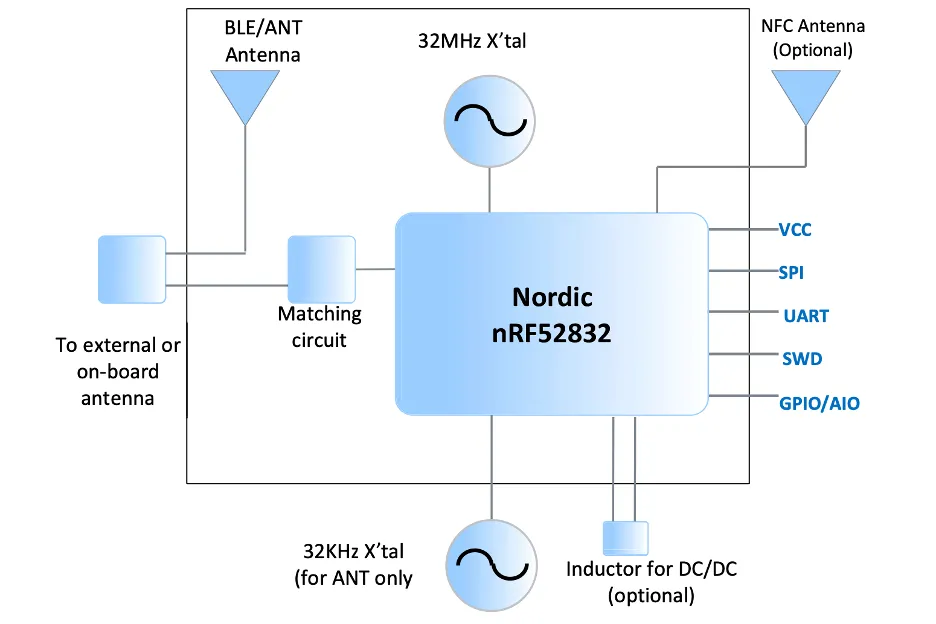
Figure 4: MBN52832 BLE 5.0 Module Block Diagram
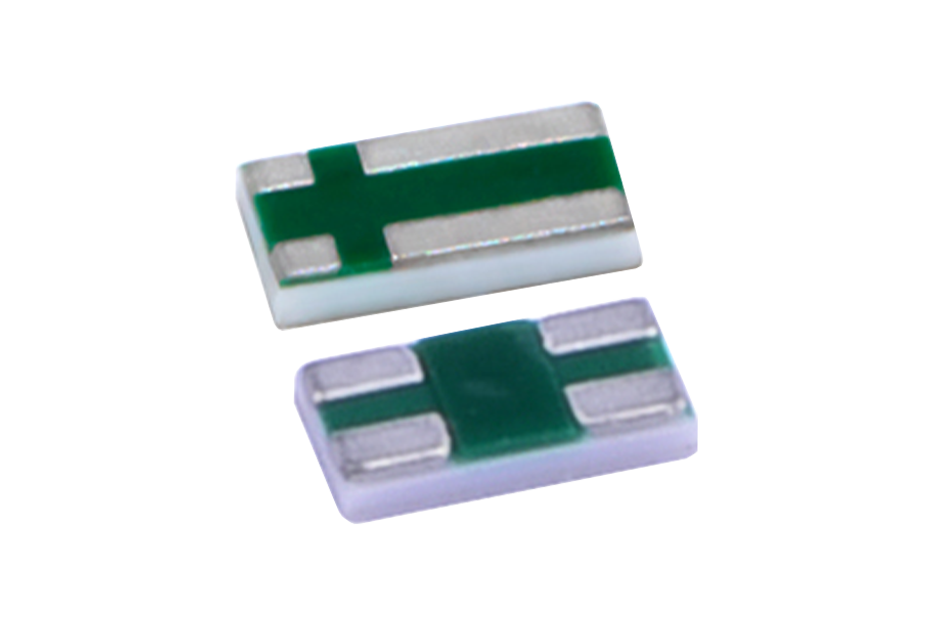
Hybrid crystal oscillator: empowering reliable intelligent control
In electronic devices, crystal resonators (referred to as crystal oscillators) are often compared to the "heart" of electronic systems, providing a stable clock reference signal for the normal operation of the system. As a medical device that concerns the health of users, the reliable and stable operation of blood glucose meters naturally requires the support of high-performance crystal oscillators.
Considering the particularity of blood glucose meter applications, in the selection of crystal oscillators, in addition to precision as a hard indicator, factors such as product appearance and cost should also be comprehensively considered.
Murata's HCR hybrid crystal resonator is a compact and cost-effective SMD resonator suitable for blood glucose meters. The so-called "hybrid" refers to the HCR crystal oscillator sealing quartz crystal elements in a reliable package used in Murata ceramic resonators. The combination of the two technologies allows the clock element to maintain quartz level accuracy (within ± 10ppm), while also having unique advantages such as smaller size and lower cost compared to standard quartz crystal oscillators.
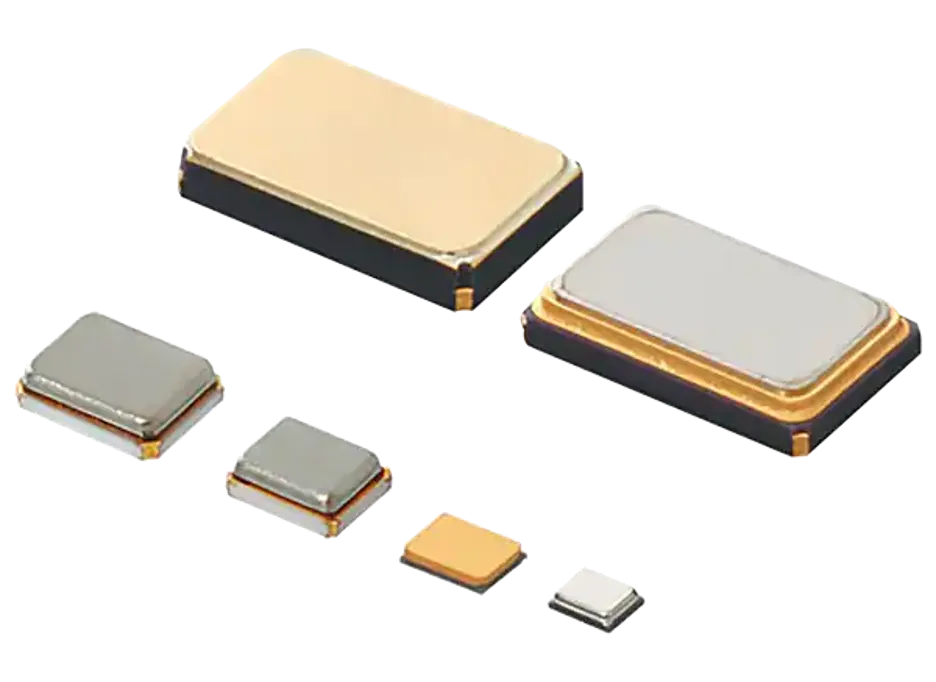
Figure 5: HCR SMD crystal oscillator
Due to the use of a universal high planar aluminum oxide substrate combined with a metal cap packaging structure, The HCR series has lower manufacturing and material costs compared to traditional crystal oscillators packaged in ceramics with hole structures, and has more stable and efficient production capacity, which is greatly beneficial for cost reduction and optimization of the blood glucose meter product supply chain.
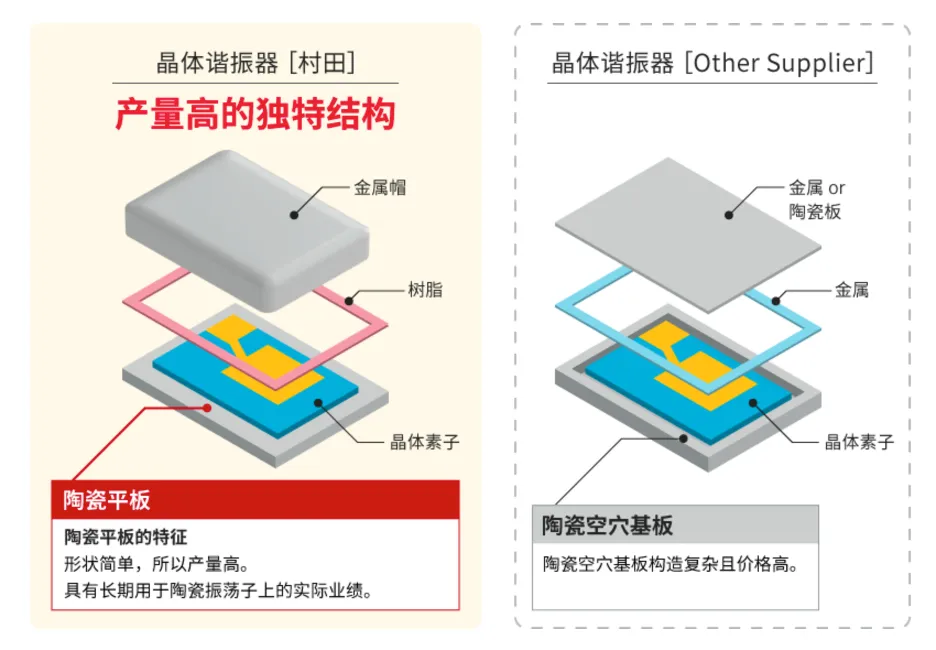
Figure 6: Advantages of the unique structure of HCR SMD crystal oscillator
In addition, The frequency variation of HCR crystal oscillator after reflow soldering is small, making it convenient for customers to compensate for frequency and supporting a wide temperature range. This enables it to provide a high-precision, high reliability, compact appearance, reasonable cost, and stable supply solution for the development of blood glucose meters, effectively empowering the intelligent control and connection needs of blood glucose meters.
NTC thermistor: providing more accurate CGM
As mentioned earlier, the currently popular CGM blood glucose meter mainly monitors glucose levels in the blood by inserting sensors into the lower layer of the skin. In principle, temperature can affect the reaction rate and enzyme activity of the glucose sensor, thereby affecting the measurement results. Generally speaking, as the temperature increases, enzyme activity increases, and the glucose reaction rate accelerates, resulting in a lower glucose level displayed by the glucose sensor; On the contrary, when the temperature decreases, the glucose level displayed by the sensor will be higher.
Therefore, in order to obtain more accurate blood glucose data, it is necessary to measure the temperature around the glucose sensor through a temperature sensor (usually an NTC thermistor), and correct the measurement results of the glucose sensor based on temperature changes to obtain more accurate and reliable measurement data.
Murata NCU series NTC thermistor is an ideal choice for the temperature sensor of CGM blood glucose meter. This thermistor has excellent performance (high precision and B constant) and excellent anti-aging stability, and can work reliably in any environment. Moreover, NCU NTC thermistors also have outstanding solderability and are compatible with fluid welding/reflow welding processes, which is very beneficial for improving production capacity and reducing overall costs. Overall, for highly reliable applications such as CGM blood glucose meters, Murata's NCU series NTC is undoubtedly the best choice.

Figure 7: NCU NTC Thermistor
Summary of this article
With the increasing number of diabetes patients worldwide, blood glucose monitoring has become a new demand and is also driving up the market demand for blood glucose meters. As a health-related medical device, blood glucose meters have unique design requirements compared to other consumer wearable products, which also raises the technical threshold for development.
To overcome these technological barriers, it is necessary to select high-performance, highly reliable components with low power consumption and cost advantages into the BOM of blood glucose meters. The three products introduced in this article are representative of them, and if you want to select more categories of ideal electronic components, Murata's overall blood glucose meter solution can definitely help you!
Note: Murata's products can only be used as a component in some health and medical products, and they do not have any disease treatment function and are not considered medical devices.